BOTOX Update: New Laws, New Products, New Opportunity!
BOTOX Update:
New Laws, New Products, New Opportunity!
From July 1st, 2023, new rules around the use of facial injectable treatments are set to take effect, coinciding with Australia receiving the first new Botox product in almost a decade. Dr Markus Weber discusses how these long-awaited developments present a BIG opportunity for Dentists.
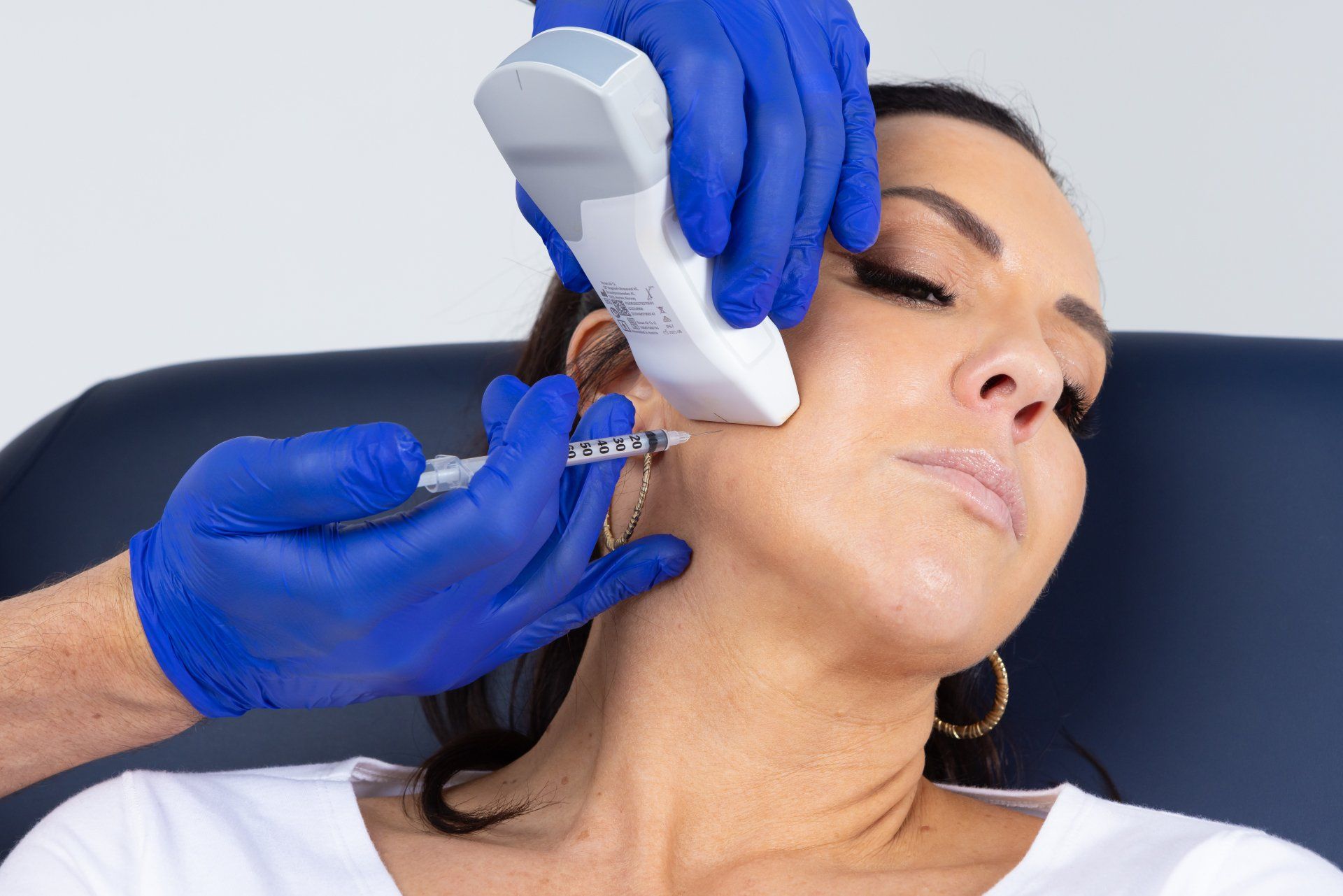
Since AADFA first introduced the concept and training in dento-facial aesthetics back in 2009, the practices and patients of thousands of Australian and New Zealand Dentists have benefitted from providing Botox and Dermal Filler injections.
Yet despite the excitement and success, practitioners and their patients also had to manage certain limitations, inherent with these products and procedures. Chief among these issues was products were expensive to buy, with that cost necessarily passed along to patients, placing it beyond the financial reach of some.
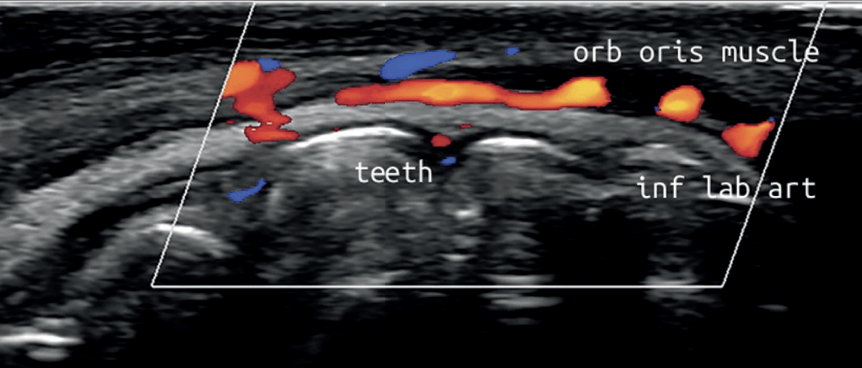
Additionally, unscrupulous practices in the broader “cosmetic” industry created difficulties for some Dentists being able to compete on an even playing field, while the risk of significant complications, like blindness from dermal filler injections, loomed large in the minds of practitioners with no direct imaging techniques available to guide them and keep them safe.
Thankfully, a long overdue review of the “cosmetic” industry has brought about sweeping changes to regulations governing the provision of Botox and Dermal Filler treatments. The new regulations commence mid-year and along with a newly launched Botox product; ultrasound guidance for injections; and enhanced treatment protocols and training programs, mean that Dentists are now better placed than ever before to take the responsible lead in the field of dento-facial aesthetics.
NEW LAWS
The “cosmetic” industry has been cowboy country for far too long, with horror stories becoming regular headlines. Thankfully, starting on July 1st , regulations are being tightened, targeting everything from levels of continuing education and the process of patient consultation, through to predatory advertising and the use of certain misleading professional titles.
AADFA fully supports these long-overdue changes and yet there has been some inaccurate commentary around the true implications of these changes for Dentists.
While the new regulations have been initiated by the Medical Board of Australia and initially focus primarily on cosmetic surgical procedures, performed by medical practitioners, there are also clear guidelines for the provision of non-surgical procedures, including Botox and Dermal Fillers. Some commentators have suggested that as these regulations are directed toward medical practitioners, they have no bearing on Dentists providing the same services – this is short-sighted.
It stands to reason that Dentists would be expected to be in line with their medical colleagues providing the same services, with the Dental Board already stating in their fact sheet on the topic that they will draw on such advice to other health practitioners, when managing and assessing a notification against a Dentist. Dentists are already expected to avoid inappropriate social media promotion and advertising which glamorises cosmetic procedures and minimises the complexity and risks of procedures, and they are already warned against the use of deliberately misleading and deceptive titles like “cosmetic injector”.
However, what’s comforting is that because of AADFA’s industry-leading approach to comprehensive education and responsible patient care from the beginning, AADFA trained Dentists are well-positioned in relation to the new rules, already representing the higher standard and following the proper protocols that regulators are now trying to replicate and enforce within the broader cosmetic industry.
In fact, the new requirements for all practitioners will now serve to level the playing field, reducing the negative impact of unscrupulous budget cosmetic chains and allowing the high standards maintained by Dentists to become the preferred option for patients and the gold-standard in the industry, increasing opportunities for Dentists to excel in this field.
However, there are a few points in the new regulations that should concern some Dentists, especially those who trained in these procedures a long time ago, or who undertook training as a basic short course, with an unrecognised provider.
1. Ongoing recognised education:
Aside from the existing Dental Board requirement for Dentists to ensure they undertake initial training at an appropriate standard, by a recognised provider, the new regulations take this further, requiring practitioners whose scope of practice includes cosmetic procedures, to undertake regular continuing education that includes activities related to those procedures and which involve reviewing their performance and measuring their outcomes.
This means that a Dentist who underwent basic training once and has done nothing since to maintain and update their knowledge, skills and competencies, will now be in breach. It is also insufficient to simply read a textbook or journal article; watch a YouTube video or webinar; or even attend a conference. Dentists are required to engage in an activity, like AADFA’s “Refresher” programs, which test and provide mentoring feedback to ensure ongoing professional competency in this field.
2. Botox and Dermal Filler are not enough:
In the last few years, we have seen the convergence of two phenomena in the cosmetic industry - patient’s increasingly seeking more natural results and the development of safer treatment options, beyond the old Botox and Dermal Filler approaches. For the first time these new regulations make it abundantly clear that Dentists can no longer simply offer Botox and Filler injections without a comprehensive understanding of the many alternate treatment options for facial rejuvenation.
While Dentists do not have to provide all of the alternate services themselves, in order to gain fully informed consent, it is now a requirement that practitioners must discuss all other treatment options in the patient consultation, including procedures that may make treatments safer and are available and offered by other practitioners.
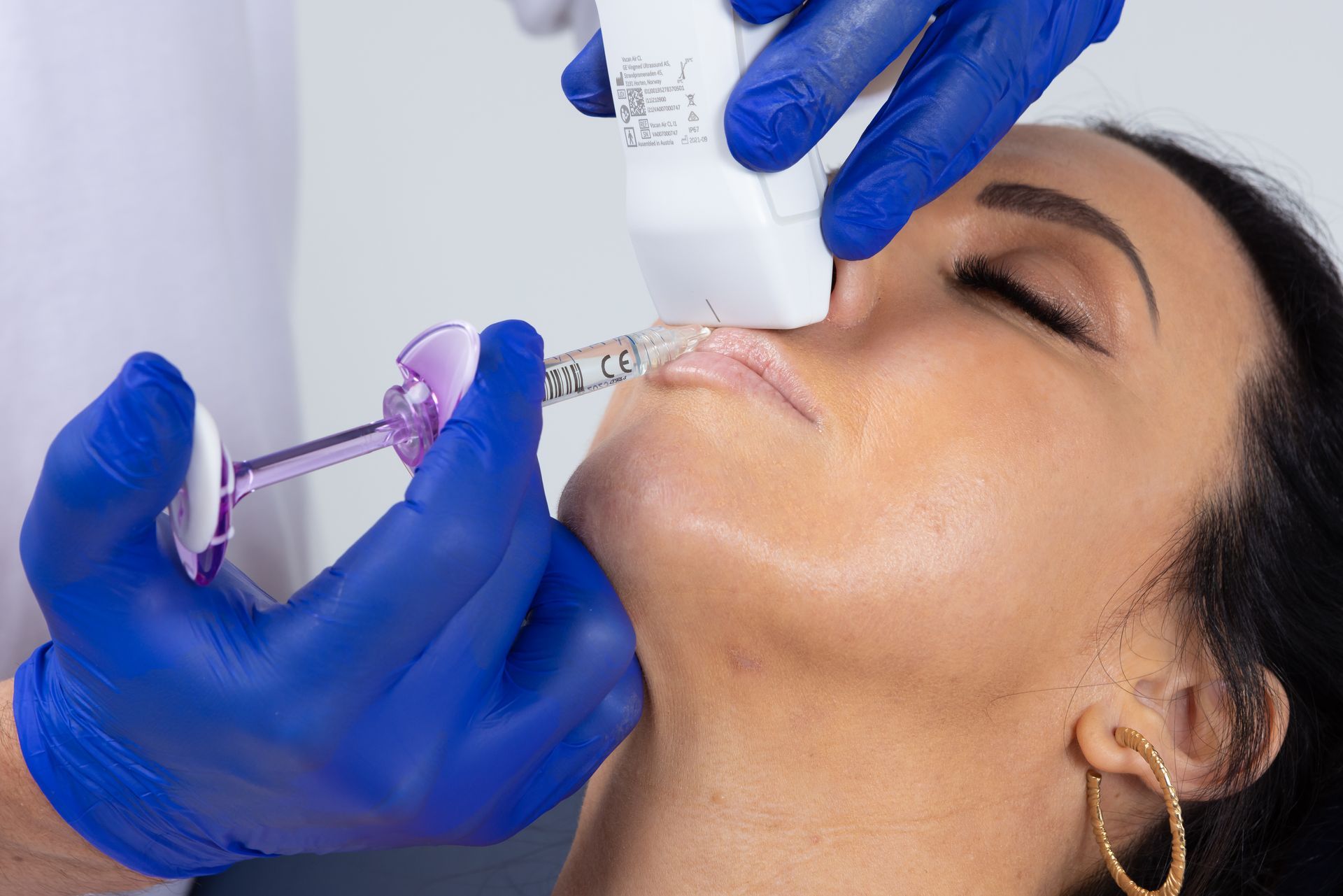
Many responsible Dentists have already recognised this inherent professional duty, engaging in AADFA’s comprehensive Fellowship Pathway of Education, becoming highly trained early adopters of new technology and treatments which offer safer, more natural and more effective options for patient care, beyond Botox and Filler. This includes AADFA Dentists currently being the number one providers of facial thread lifting procedures and the number one users of portable ultrasound technology, allowing them to reduce their reliance on older, more dangerous materials and - now able to directly visualise anatomy in real-time - eliminate complications from dermal filler, like blindness and skin death. This is also in line with the existing National Safety and Quality Health Service (NSQHS) requirement for practitioners to minimise risk to patients.
3. Psychological Screening:
In todays’ social media driven world, it is an unfortunate fact that an increasing number of patients, of all genders and ages, seek cosmetic treatments in response to feelings of inadequacy and negativity, in the hope that they will somehow find an inner peace through external enhancement.
While well-trained Dentists are already aware of this growing potential, and include an assessment of common signs associated with these psychological conditions in their consultations, new regulations now make it compulsory for practitioners offering cosmetic treatments to specifically screen for conditions like Body Dysmorphic Disorder (BDD), using a recognised and appropriate screening tool.
If the screening tool indicates that the patient may have underlying psychological issues making them unsuitable for the cosmetic procedure, they must be referred for evaluation to a psychologist, psychiatrist or general practitioner who, importantly, does not provide cosmetic procedures themselves.
NEW PRODUCTS
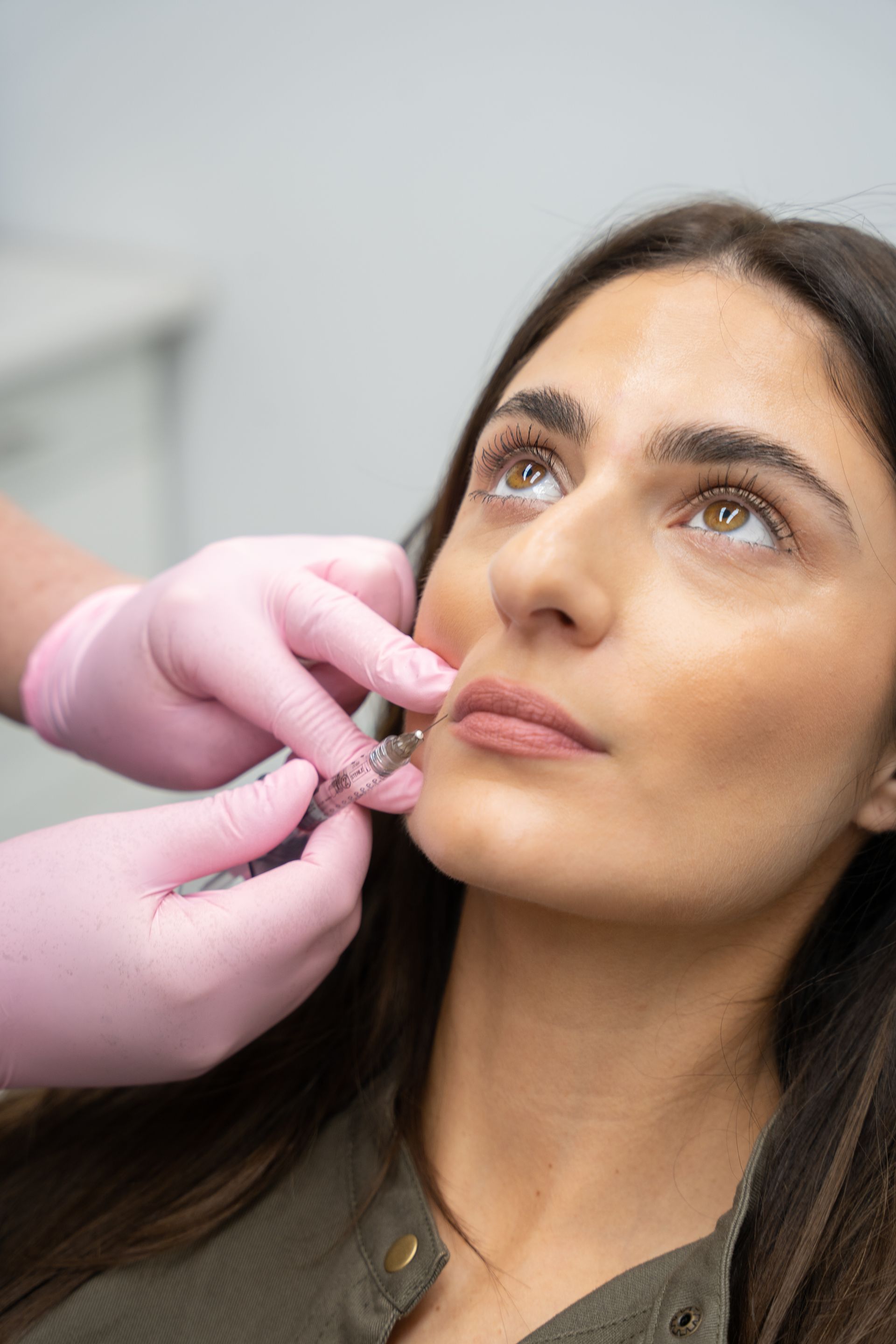
One of the issues facing practitioners entering the facial injectable arena in Australia has traditionally been the high cost and limited choice of available Botulinum Toxin (Botox) products. This was also a major concern and obstacle for many patients, coupled with a limited treatment longevity of around 3-4 months. Despite numerous products being available overseas, and many more under development, this lack of competition in the marketplace remained frustrating for local patients and practitioners for many years, with it being close to a decade since the last new Botox product arrived.
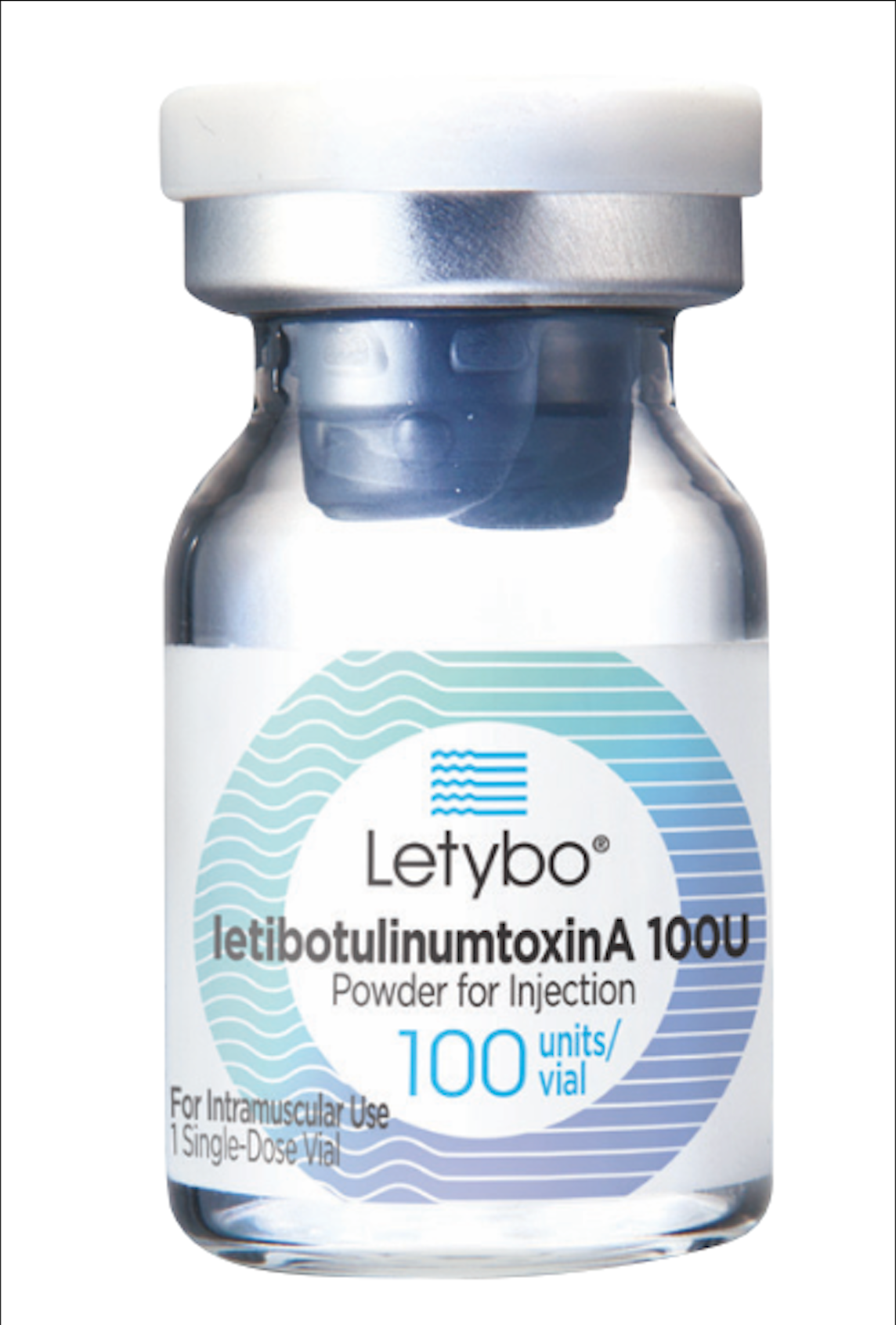
This status quo is set to change thanks to a flurry of new products entering the market in coming years, starting with Letibotulinum Toxin (Letybo®, Hugel Aesthetics), which is currently the only new product available to Australian practitioners right now.
Letybo® is a product that dilutes; has a unit system; and injects in line with that of the current Australian market leader, Botox®. While new to the Australian market, it has a long history, having been developed in South Korea over 12 years ago; registered, used and extensively researched in over 50 countries worldwide; with over 26 million vials being used to date.
However, the first important thing to note is that none of these new products will be “game changers”. A game changer would denote a product that somehow revolutionised the way the treatment was delivered (e.g. a topical versus injectable); making it more simple; improving the safety profile of the treatment; or that delivered spectacularly better results that the others couldn’t – none of these new products do any of that. What they do is increase industry competition and drive down prices.
New products fall broadly into one of two categories: A) those that deliver exactly the same treatments we are all used to, but at a much lower cost for practitioner and patient (e.g. Letybo® and Jeavenu®, Evolus), or, B) those that claim to last longer but are considerably more expensive, difficult and different to use (e.g. Daxxify®, Revance Aesthetics). AADFA believes that the lower cost predictable products will better drive industry competition that has been long overdue, ultimately benefitting our patients financially and making them accessible to more people - but they won’t change the clinical landscape at all.
While the clinical landscape could potentially be changed by longer-lasting products, which certainly sound appealing, what some who have drunk the cool-aid and swallowed the marketing hype of product manufacturers, describing them as “game changers”, simply fail to understand, is that products rarely behave in the real world as they do in sponsored research projects.
To date, this category of products is largely untested globally in real world settings. Practitioners in a single country have only been using this product for less than 6 months and all reports are that, while the thought of (maybe) achieving a month or two longer clinical duration is appealing, it is substantially outweighed by the higher cost of each treatment and the fact that the product requires practitioners to operate completely differently to how they have been working for years.
Perhaps most importantly, even if the longer duration does prove to be a reality, this would also mean that any potential complications experienced would also last considerably longer – 6 to 9 months of a drooping eyebrow or asymmetrical smile hardly sounds appealing to many patients. Given the fact that the first new product to hit the Australian market in a decade is easy to use, requires no change in behaviour, presents no new risks, and will drive down consumer pricing, it’s hard to see a day when a more expensive, more complex product will significantly move the needle (pardon the pun), just in the hope that it may last a little longer.
Want to know more?
AADFA recently held a series of webinars with in-depth discussion on the new regulations and product developments, including information on the best psychological screening tools to use in clinic during cosmetic consultations.
For further information, and to ensure you receive the best possible ongoing training and resources to remain compliant, contact AADFA for a recording of those sessions by visiting www.AADFA.net or emailing clinical@AADFA.net.