Injecting Botox & Dermal Filler the old way? Stop now!
AADFA INTERNATIONAL discusses the new clinical & legal standard that has now commenced for practitioners performing facial aesthetic procedures.
Coinciding with the development of new technology and clinical protocols for the delivery of Botox, Dermal Filler
and other facial aesthetic procedures, several new regulatory and legal standards, concerning dental practitioners, took effect this month. The upshot for Dentists performing facial aesthetic procedures is – continue injecting the old way at your own peril!
What was wrong with the old way of injecting Botox and Dermal Filler?
In almost every aspect of modern dental practice, clinical procedures are made safer and more effective by using routine imaging.
Whether it be digital or conventional film; OPG, bitewing, periapical or CBCT; the use of some reliable method of imaging is considered best practice for all manner of dental treatments, from implants, through to extractions and root canal. Indeed, the failure of a dental practitioner to properly utilize available imaging to guide diagnosis and treatment delivery, essentially flying blind, would be considered a substantial departure from accepted standards, exposing them to regulatory action and litigation.
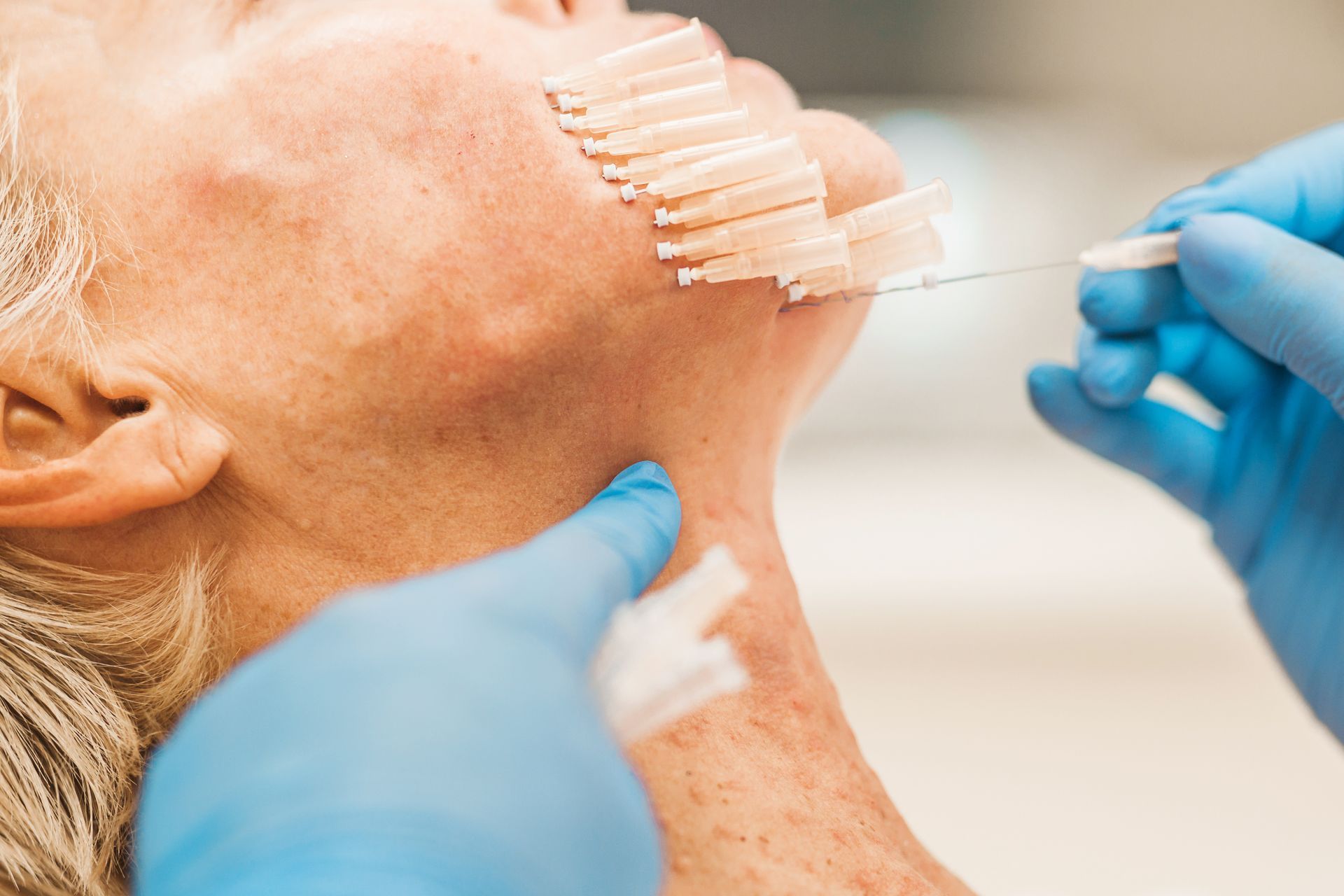
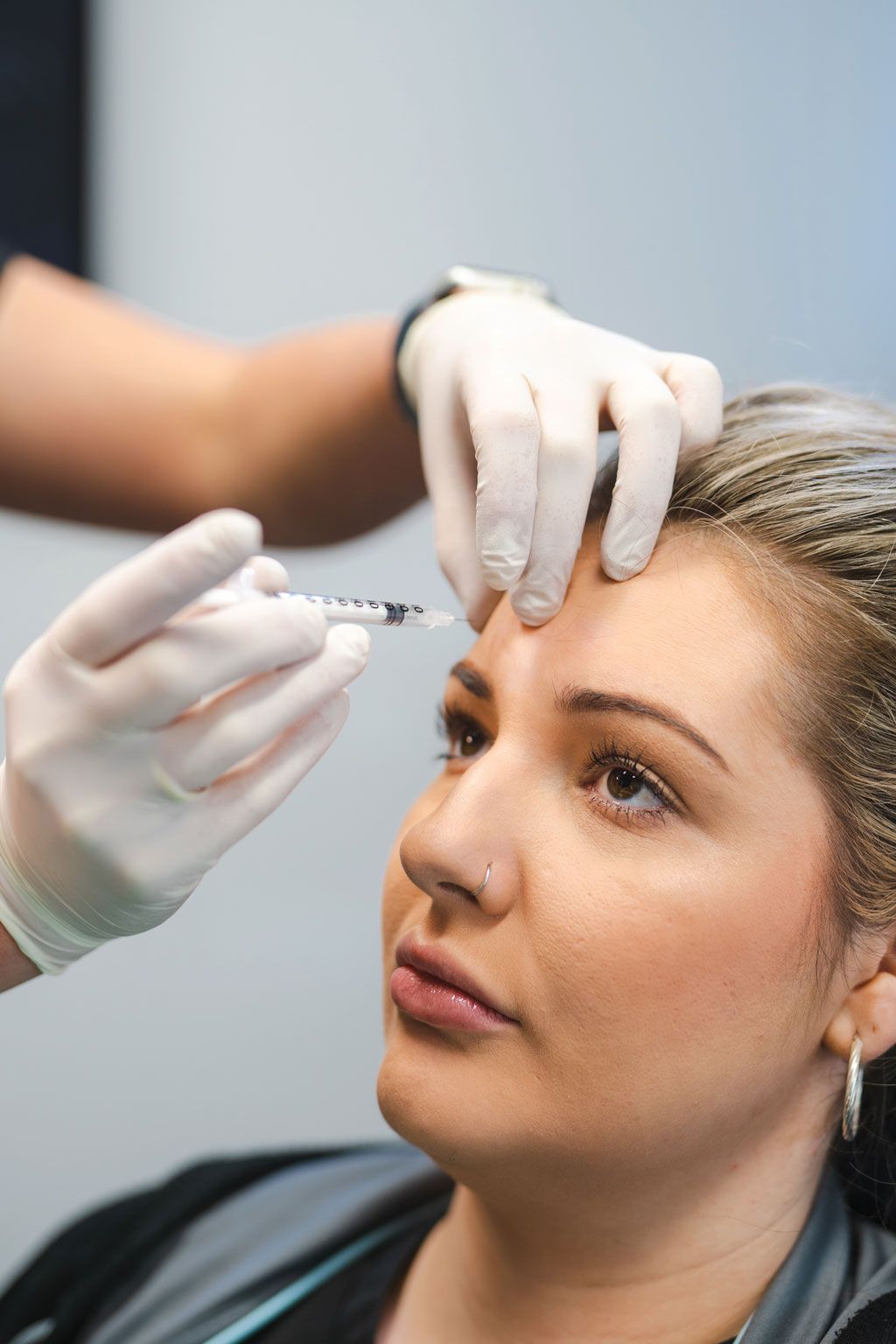
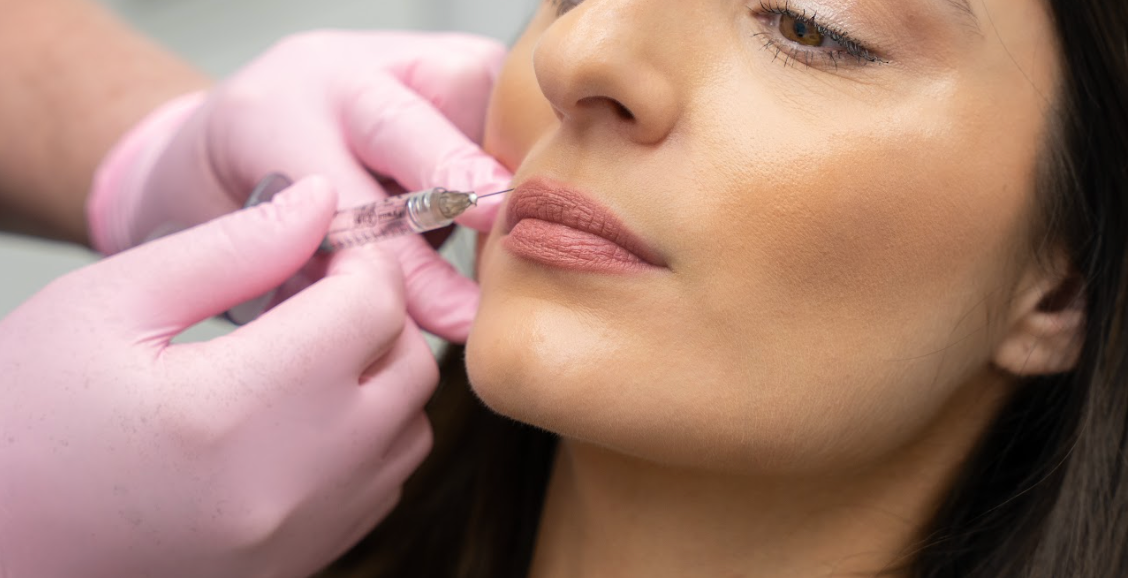
Yet, until recently, a blind approach to treatment provision was the only option practitioners had when performing facial aesthetic procedures like Botox and Dermal Filler, as no appropriate chairside imaging method was available. This meant that, no matter how experienced a practitioner was; how well they understood facial anatomy; or how they tried to deploy supposedly “safer” techniques; the extreme variation and complexity of individual facial anatomy made encountering complications and poor clinical outcomes practically inevitable at some point.
While, thankfully, serious complications from Botox and Dermal Filler are rare, they have been increasing dramatically as the popularity of such treatments grows exponentially. Given the devastating potential of some of these complications; ranging from scarring, permanent disfigurement, blindness and stroke; even a low risk means that practitioners are ethically and legally obliged to utilise safer methods as they become available.
What’s changed clinically?
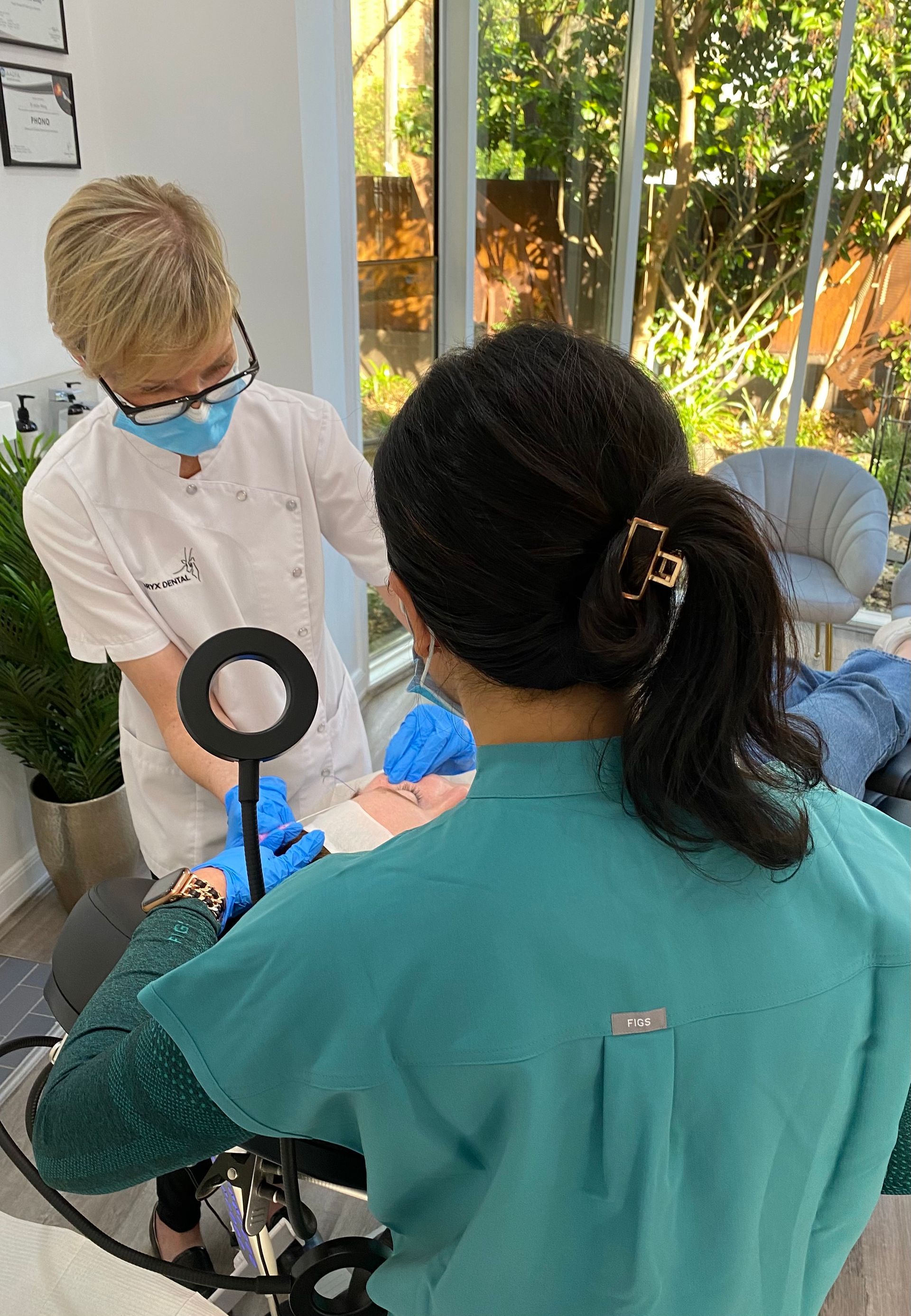
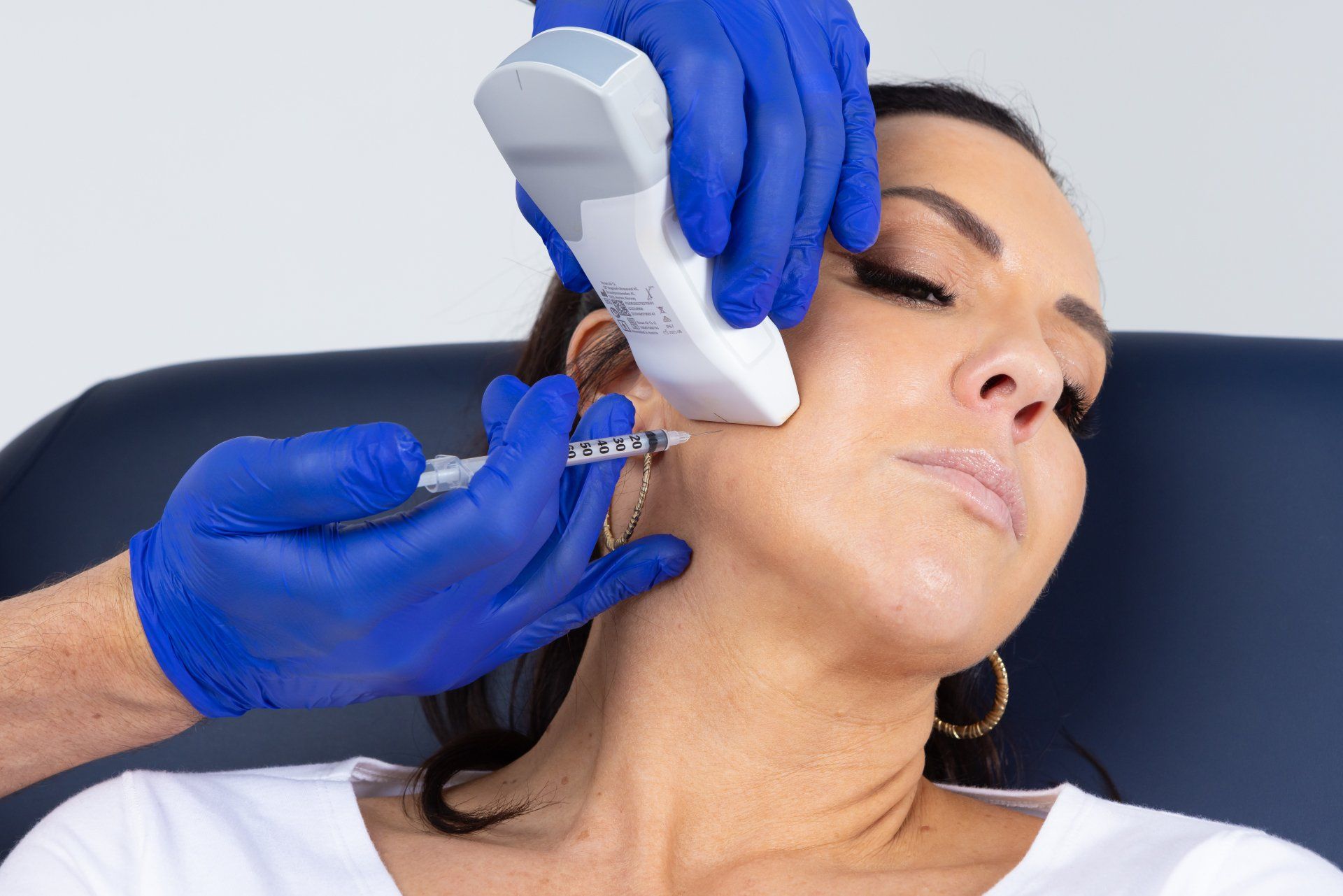
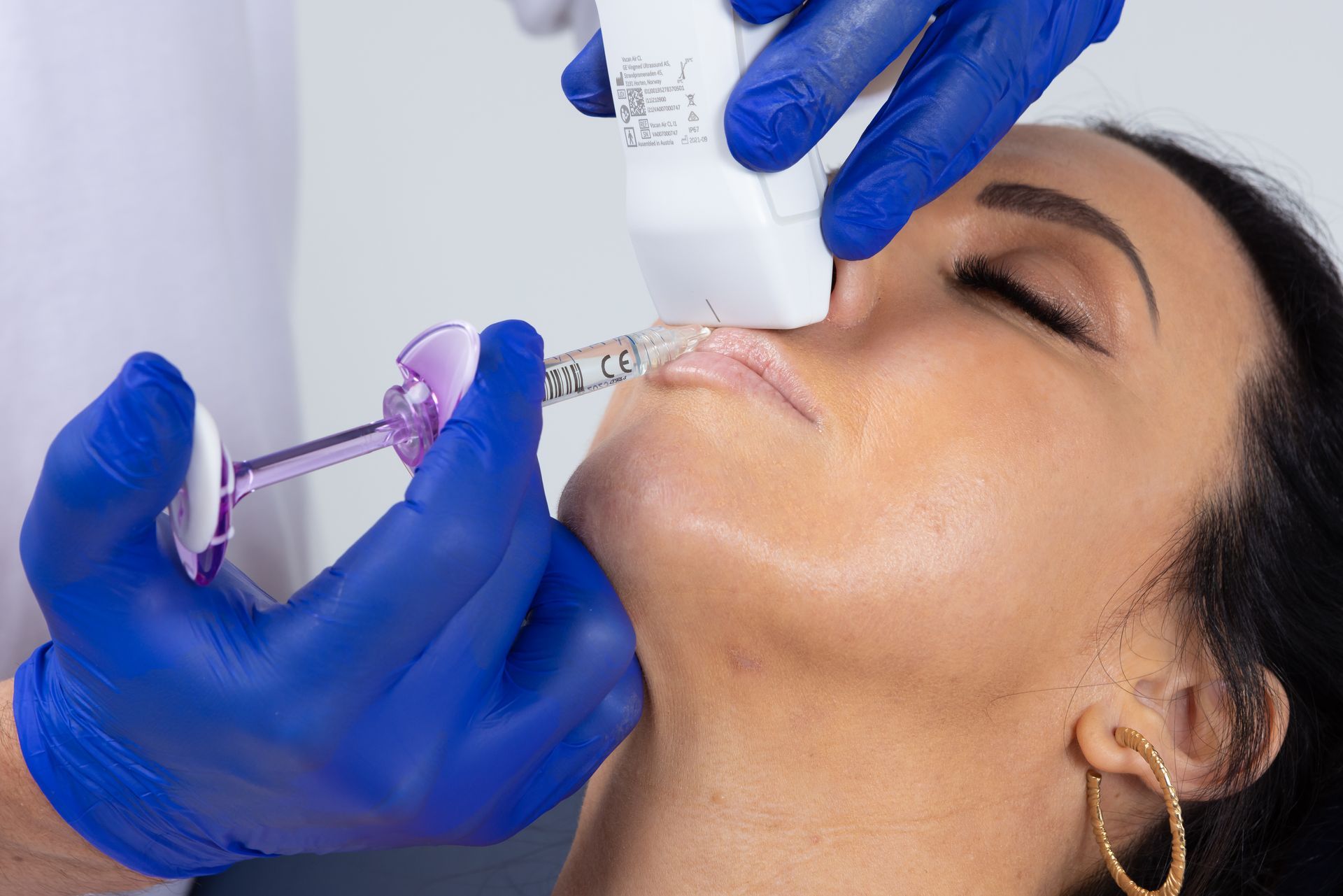
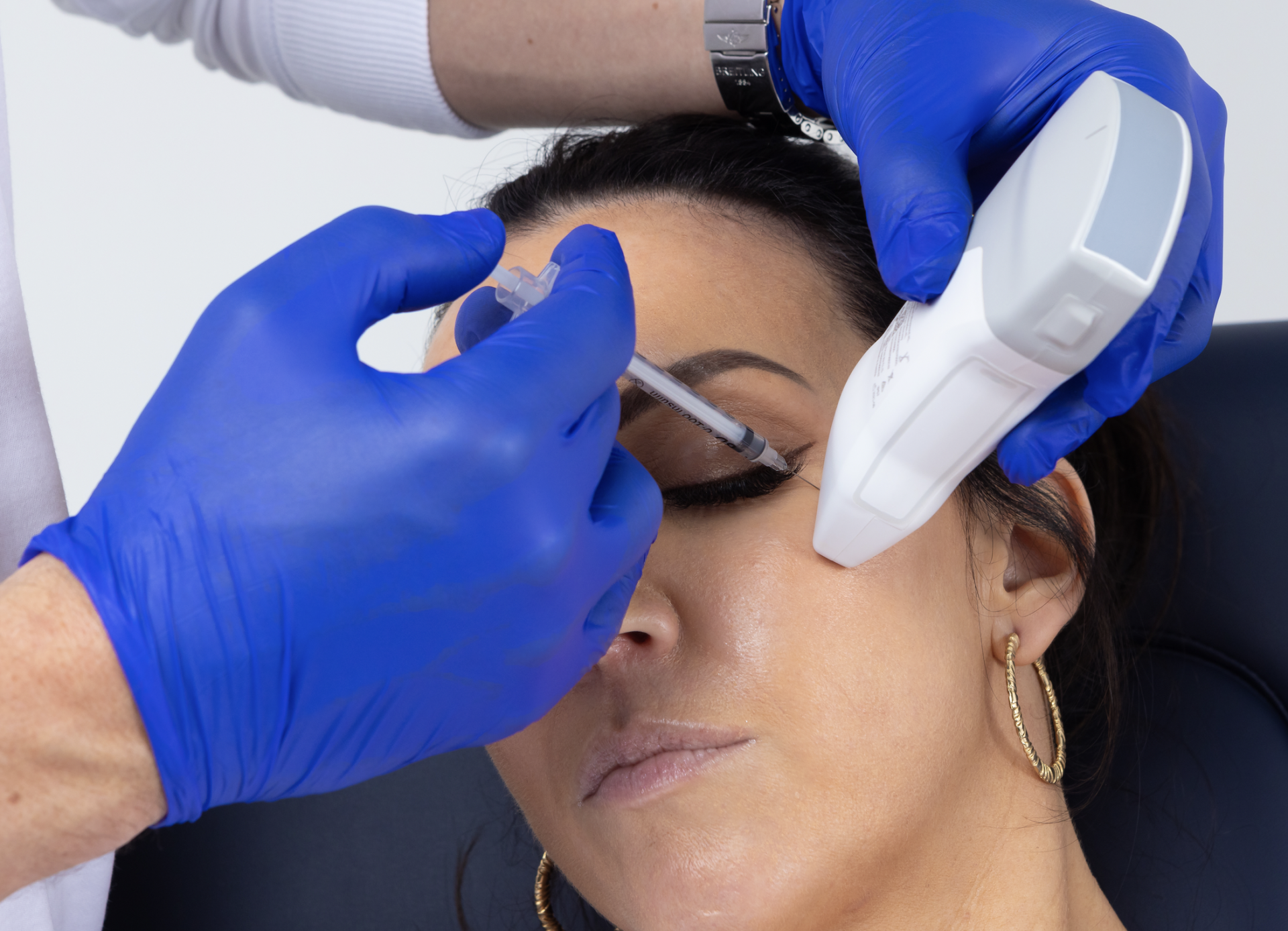
Ultrasound technology has been recognised for decades as being a reliable method for accurately assessing the anatomy of various soft tissues and guiding injection procedures in numerous fields of medicine. However, until recently, ultrasound devices were cumbersome, difficult to operate and prohibitively expensive, making point- of-care use in a dental clinic unviable. This has all changed with the advent of the latest portable, wireless, hand-held, ultrasound technology, together with the establishment of clear and simple clinical protocols for guided Botox and Dermal Filler injections.
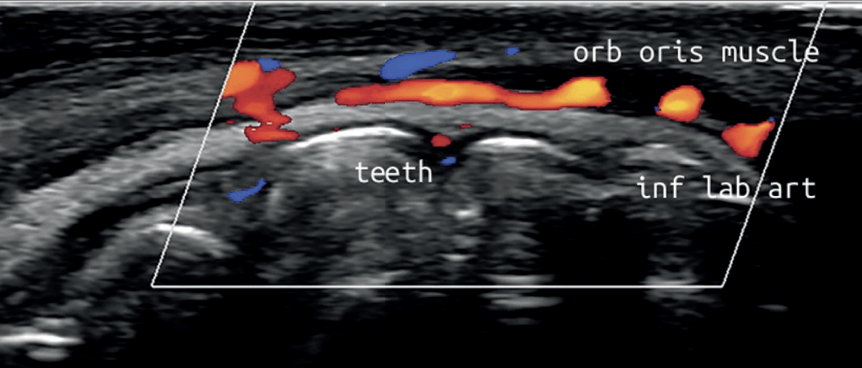
These new low-cost devices; costing less than a standard dental x-ray unit and being no bigger than an iPhone; are able to deliver real-time, high-quality diagnostic images direct to a tablet or smartphone, painlessly and seamlessly, at chairside, without ionising radiation. With special settings allowing for the colourful visualisation of blood vessel location, size and velocity of flow, Dentists now have an imaging tool and protocols allowing them to diagnose and pre-plan like never before; as well as visualising precise target tissues for injection; danger areas to avoid; and to be guided in their treatment delivery, eliminating the risky blind injecting of the past.
What are the legal and regulatory implications?
With the introduction of ultrasound imaging protocols for Botox and Dermal Filler injections, facial procedures have now been brought into line with the long- standing norms of traditional dentistry. As such, the revised Code of Conduct for Dental Practitioners launched this month by AHPRA; together with the recently released position statements and standards of the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) and the National Safety and Quality Health Service (NSQHS); make the obligations of dentists and the expectations of regulators, abundantly clear.
In relation to the new Dental Board Code of Conduct, AHPRA has stated the Board “will use this code when evaluating practitioners’ professional conduct,” where a failure to meet the code, risking patient harm, could have “consequences for registration”. The new code states that dental practitioners “should practise safely” and “make responsible and effective use of the resources available to practitioners” (Principle 1).
The Code states that, “Good practice involves putting patient safety ... first. Practitioners should minimise risk by ... applying the principles of clinical governance, risk minimisation and management in practice.” (Principle 7). Notably, NSQHS states clearly that effective clinical governance involves taking all available and appropriate steps to eliminate, reduce and manage the risk to patients in order to achieve good clinical outcomes.
Following the code, dental practitioners are expected to, “develop and implement risk management processes that identify and minimise risk to reduce harm to patients”, and they should, “work to reduce error and improve patient safety”. On this point, the EFSUMB has stated clearly that, “Percutaneous ultrasound guidance of non-surgical aesthetic procedures is recommended as it increases precision and decreases the potential for unwanted effects or neighbouring injuries”.
So, in summary, failure to utilise an easily accessible imaging protocol during Botox and Dermal Filler procedures, which represents the new professional standard, would fall substantially below the level of care expected of a responsible dental practitioner. Continuing to inject blindly, would expose practitioners to regulatory action and even patient litigation in the event of sub-optimal outcomes or complications emerging that could otherwise have been avoided. Additionally, a failure to disclose to patients that a method of visualisation exists which would make procedures safer and more effective, would undoubtedly mean a failure to gain informed consent, in breach of Principle 4 of the Code of Conduct.
Where can I find out more?
To assist dental practitioners understand their responsibilities in relation to the new clinical and legal standard for injecting Botox and Dermal Filler, AADFA International hosted a series of FREE webinars. TO VIEW the series CLICK HERE
Proudly Supported By:

Contact Us
We will get back to you as soon as possible.
Please try again later.
© 2009-2024. All Rights Reserved. Australasian Academy of Dento-Facial Aesthetics.